Summary
この調査レポートでは、RPM市場を支える機器と技術について詳細に調査・分析しています。RPMに使用されるデバイスは多岐にわたり、体重計や血圧計などの確立された技術から、外来心臓モニタリング(例:モバイル心臓テレメトリ)や連続グルコースモニタリングなどの成長セグメント、さらには消費者向けウェアラブルでバックグラウンドでモニタリングできる健康指標の数を増やすための現在の取り組みまで、多岐にわたります。
主な掲載内容(目次より抜粋)
Report Summary
As healthcare spending around the world increases, innovative methods of delivering cost-effective healthcare must be developed. Remote patient monitoring (RPM), referring to the use of technology to measure and transmit physiologic data beyond traditional healthcare settings, has emerged as a promising solution to drive down healthcare costs while maintaining a high standard of care for patients. The importance and value of RPM has been emphasized by the need to keep patients out of hospitals and clinics during the COVID-19 pandemic, which has in turn accelerated adoption of RPM.
Source IDTechEx
Overall, RPM technologies and services enable healthcare providers to care for their patients outside of their regularly scheduled visits, following a global trend in decentralization to alleviate overburdened hospitals and clinics. For patients, RPM not only allows for the elimination of costly and time-consuming travel, but also increases quality of life, reduces hospitalizations and emergency room visits, and reduces hospital stay times. Early warning signs may be detected in time for preventative healthcare to be administered.
Today, the market for RPM technologies and services focuses on key chronic conditions spanning cardiovascular disease, diabetes, and respiratory diseases, particularly in older adults. The world's elderly population is responsible for a large portion of healthcare spending due to the high prevalence of chronic diseases in this group, and the high cost of managing chronic diseases. Management involves high levels of care (regular check-ups and good adherence to medication regimen) over long term. With the increase in the world's elderly population predicted, even small cost reductions in this space can have drastic impacts on healthcare systems around the world.
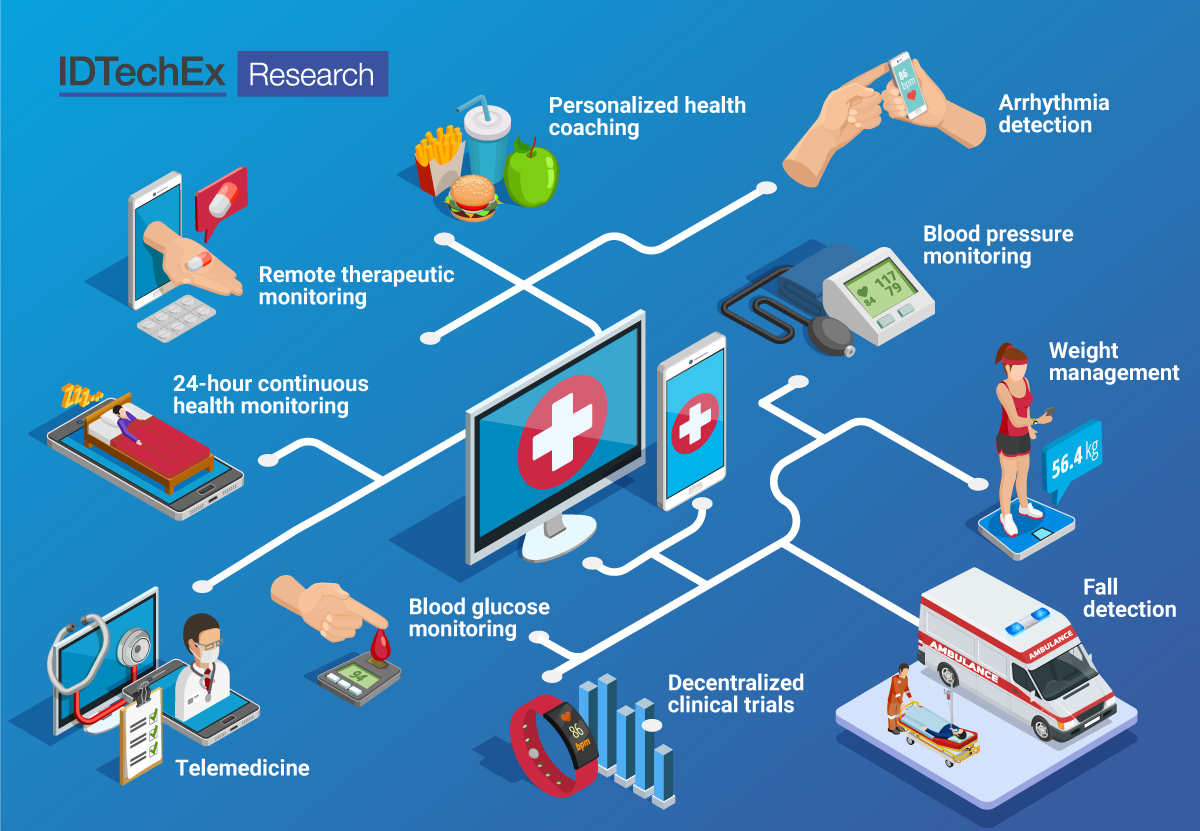
This IDTechEx report focuses on the devices and technologies supporting the RPM market. The range of devices used in RPM is vast, spanning established technologies such as scales and blood pressure cuffs to growing segments such as ambulatory cardiac monitoring (e.g. mobile cardiac telemetry) and continuous glucose monitoring, to current efforts to increase the number of health metrics that can be monitored in the background on consumer wearables. Innovations in RPM technology focus on increasing patient comfort, convenience, and adherence to monitoring, while maintaining high data quality, and where relevant, providing the option of continuous monitoring. Recent activities from medical and technology giants show their interest in entering the RPM market, bringing with them much needed scale needed to support uptake. Increased reimbursement and both patient and physician comfort with the technology (due to the COVID-19 pandemic) can support the continued rise of RPM technologies.
In this report, IDTechEx leverages our coverage of wearable and medical technology to bring the latest in technologies, applications, and opportunities for RPM. The report takes a deep dive into a spectrum of RPM approaches in the following high impact areas:
-
Use of electronic skin patches and non-contact monitoring systems to track patients' vital signs for detection of deterioration
-
Development of cuffless blood pressure monitoring devices for management of hypertension
-
Shift towards consumer technologies in arrhythmia detection, and increasing wear time and patient comfort in mobile cardiac telemetry
-
Increasing adoption of continuous glucose monitoring technology along with efforts to closed loop insulin delivery
-
Establishment of novel digital biomarkers and adoption of RPM for decentralized clinical trials
-
Use of sensors around the home for monitoring the elderly for activities of daily living and falls
-
Potential for wearable wellness devices as an incentive for health insurance
Examples of technologies covered in the report include electronic skin patches, smart shirts, smartwatches, and connected inhalers. This report encompasses IDTechEx's expert analysis of wearable technology, wearable sensors, electronic textiles, electronic skin patches, and digital health, to bring to the reader the most relevant insights on the exciting innovations in RPM.
Key aspects
This report provides the following information:
-
Overview of the remote patient monitoring industry, including key companies and key industry drivers
-
Analysis of technology and trends, including key chronic disease applications
-
Increasing patient comfort and bringing continuous monitoring to hospital wards
-
Hypertension and the development of cuffless blood pressure
-
Heart rhythm and the rise of wearables
-
Diabetes and the push towards continuous glucose monitoring
-
Opportunities for wearables in movement disorders
-
Monitoring the elderly
-
Digital biomarkers and decentralized clinical trials
-
Health insurance and incentives for wearable technology
-
10-year market forecasts for connected medical devices for remote patient monitoring of chronic conditions, spanning 19 products / product categories
Enable GingerCannot connect to Ginger Check your internet connection
or reload the browserDisable in this text fieldRephraseRephrase current sentenceEdit in Ginger
Enable GingerCannot connect to Ginger Check your internet connection
or reload the browserDisable in this text fieldRephraseRephrase current sentenceEdit in Ginger
Enable GingerCannot connect to Ginger Check your internet connection
or reload the browserDisable in this text fieldRephraseRephrase current sentenceEdit in Ginger
Enable GingerCannot connect to Ginger Check your internet connection
or reload the browserDisable in this text fieldRephraseRephrase current sentenceEdit in Ginger
Enable GingerCannot connect to Ginger Check your internet connection
or reload the browserDisable in this text fieldRephraseRephrase current sentenceEdit in Ginger
ページTOPに戻る
Table of Contents
Enable Ginger
|
1. |
EXECUTIVE SUMMARY |
|
1.1. |
Scope of the Report |
|
1.2. |
Factors Encouraging the Rise of Digital Health and Remote Patient Monitoring |
|
1.3. |
Benefits of Remote Patient Monitoring |
|
1.4. |
Components of a Remote Monitoring System |
|
1.5. |
Common Measurements and Applications |
|
1.6. |
Types of RPM Companies |
|
1.7. |
Vital Sign Monitoring In and Out of the Hospital |
|
1.8. |
RPM of Cardiovascular Diseases |
|
1.9. |
RPM for Hypertension (High Blood Pressure) |
|
1.10. |
RPM for Atrial Fibrillation & Other Arrythmias |
|
1.11. |
RPM for Heart Failure |
|
1.12. |
RPM of Diabetes and Prediabetes |
|
1.13. |
RPM of Respiratory Conditions |
|
1.14. |
RPM Technologies for Other Chronic Diseases |
|
1.15. |
RPM in Clinical Trials |
|
1.16. |
Remote Monitoring of the Elderly |
|
1.17. |
Wearables for Health Insurance |
|
1.18. |
Market Forecast 2023-2033: RPM Devices |
|
2. |
INTRODUCTION |
|
2.1. |
Scope of the Report |
|
2.2. |
Factors Encouraging the Rise of Digital Health and Remote Patient Monitoring |
|
2.3. |
Changing Demographics Require Healthcare Reforms |
|
2.4. |
Global Healthcare Spending is Rising |
|
2.5. |
Why Telemedicine? |
|
2.6. |
Driving the Uptake of Telemedicine |
|
2.7. |
Benefits of Remote Patient Monitoring |
|
2.8. |
Components of a Remote Monitoring System |
|
2.9. |
Common Measurements and Applications |
|
2.10. |
The Impact of COVID-19 on Digital Health |
|
2.11. |
Shifts Towards Value-Based Care |
|
2.12. |
Positive Trends in Reimbursement Continues in the US |
|
2.13. |
From Connected to Wearable |
|
2.14. |
Broadband Access is a Determinant of Health |
|
2.15. |
Design Challenges for RPM |
|
3. |
REMOTE PATIENT MONITORING |
|
3.1. |
Overview |
|
3.1.1. |
Types of RPM Companies |
|
3.1.2. |
Teladoc Health |
|
3.1.3. |
Livongo by Teladoc Health |
|
3.1.4. |
Amwell |
|
3.1.5. |
MDLive |
|
3.1.6. |
Omada Health |
|
3.1.7. |
Optimize Health |
|
3.1.8. |
Market Outlook |
|
3.2. |
Vital Sign Monitoring - Inpatient and Outpatient |
|
3.2.1. |
Introduction |
|
3.2.2. |
Inpatient Monitoring: Background |
|
3.2.3. |
RPM in the Hospital |
|
3.2.4. |
The Case for Removing the Wires |
|
3.2.5. |
RPM in the Hospital: Form Factors |
|
3.2.6. |
Parameter Comparison |
|
3.2.7. |
The Case Against RPM |
|
3.2.8. |
Vital Sign Monitoring at Home |
|
3.2.9. |
Post-Discharge Monitoring |
|
3.2.10. |
COVID-19 at Home |
|
3.2.11. |
Cancer |
|
3.2.12. |
Cancer: Symptom Monitoring |
|
3.2.13. |
Cancer: Performance Status |
|
3.2.14. |
Deployment Challenges for Monitoring Devices |
|
3.2.15. |
Summary and Outlook |
|
3.2.16. |
Emerging Options for General Vital Sign Monitoring |
|
3.2.17. |
Summary |
|
3.2.18. |
Vital Sign Monitoring: Key Companies |
|
3.2.19. |
Electronic Skin Patches |
|
3.2.20. |
Philips |
|
3.2.21. |
The Surgical Company (formerly Sensium) |
|
3.2.22. |
BioIntelliSense |
|
3.2.23. |
VitalConnect |
|
3.2.24. |
Vivalink (VivaLNK) |
|
3.2.25. |
Non-Contact Monitoring Technologies |
|
3.2.26. |
EarlySense |
|
3.2.27. |
EarlySense |
|
3.2.28. |
Xandar Kardian |
|
3.2.29. |
Oxehealth |
|
3.3. |
Cardiovascular Diseases - Hypertension, Atrial Fibrillation, and Heart Failure |
|
3.3.1. |
Background |
|
3.3.2. |
Remote Patient Monitoring in Cardiovascular Diseases |
|
3.3.3. |
Technology Summary |
|
3.3.4. |
Electrocardiography (ECG, or EKG) |
|
3.3.5. |
Photoplethysmography (PPG) |
|
3.3.6. |
Example Charts |
|
3.4. |
Hypertension |
|
3.4.1. |
Background |
|
3.4.2. |
Classification |
|
3.4.3. |
Management |
|
3.4.4. |
Remote Patient Monitoring |
|
3.4.5. |
Oscillometric Method |
|
3.4.6. |
Oscillometric Devices |
|
3.4.7. |
Omron Healthcare |
|
3.4.8. |
Omron Healthcare: HeartGuide |
|
3.4.9. |
Omron Healthcare: VitalSight |
|
3.4.10. |
Withings |
|
3.4.11. |
Optical Measurement Techniques - Cuff-Less Blood Pressure Monitoring |
|
3.4.12. |
Pulse Arrival Time, Pulse Transit Time, and Pulse Wave Velocity |
|
3.4.13. |
Asus: VivoWatch BP, VivoWatch SP |
|
3.4.14. |
Asus: VivoWatch SP |
|
3.4.15. |
Pulse Wave Analysis |
|
3.4.16. |
Pulse Wave Analysis: Methods |
|
3.4.17. |
Aktiia: Aktiia Bracelet |
|
3.4.18. |
Biospectal |
|
3.4.19. |
Valencell: Cuffless Blood Pressure |
|
3.4.20. |
Summary and Outlook |
|
3.5. |
Atrial Fibrillation and Other Arrythmias |
|
3.5.1. |
Background |
|
3.5.2. |
(Early) Detection of Atrial Fibrillation |
|
3.5.3. |
Detection and Diagnosis of Atrial Fibrillation and other Arrhythmias |
|
3.5.4. |
Atrial Fibrillation: Screening & Detection |
|
3.5.5. |
AliveCor |
|
3.5.6. |
AliveCor: KardiaMobile |
|
3.5.7. |
Qompium: FibriCheck |
|
3.5.8. |
ページTOPに戻る
本レポートと同じKEY WORD()の最新刊レポート
- 本レポートと同じKEY WORDの最新刊レポートはありません。
よくあるご質問
IDTechEx社はどのような調査会社ですか?
IDTechExはセンサ技術や3D印刷、電気自動車などの先端技術・材料市場を対象に広範かつ詳細な調査を行っています。データリソースはIDTechExの調査レポートおよび委託調査(個別調査)を取り扱う日... もっと見る
調査レポートの納品までの日数はどの程度ですか?
在庫のあるものは速納となりますが、平均的には 3-4日と見て下さい。
但し、一部の調査レポートでは、発注を受けた段階で内容更新をして納品をする場合もあります。
発注をする前のお問合せをお願いします。
注文の手続きはどのようになっていますか?
1)お客様からの御問い合わせをいただきます。
2)見積書やサンプルの提示をいたします。
3)お客様指定、もしくは弊社の発注書をメール添付にて発送してください。
4)データリソース社からレポート発行元の調査会社へ納品手配します。
5) 調査会社からお客様へ納品されます。最近は、pdfにてのメール納品が大半です。
お支払方法の方法はどのようになっていますか?
納品と同時にデータリソース社よりお客様へ請求書(必要に応じて納品書も)を発送いたします。
お客様よりデータリソース社へ(通常は円払い)の御振り込みをお願いします。
請求書は、納品日の日付で発行しますので、翌月最終営業日までの当社指定口座への振込みをお願いします。振込み手数料は御社負担にてお願いします。
お客様の御支払い条件が60日以上の場合は御相談ください。
尚、初めてのお取引先や個人の場合、前払いをお願いすることもあります。ご了承のほど、お願いします。
データリソース社はどのような会社ですか?
当社は、世界各国の主要調査会社・レポート出版社と提携し、世界各国の市場調査レポートや技術動向レポートなどを日本国内の企業・公官庁及び教育研究機関に提供しております。
世界各国の「市場・技術・法規制などの」実情を調査・収集される時には、データリソース社にご相談ください。
お客様の御要望にあったデータや情報を抽出する為のレポート紹介や調査のアドバイスも致します。
|
|