先進創傷ケア技術 2020-2030年:診断、治療、モニタリング、慢性非治癒性創傷の市場機会Advanced Wound Care Technologies 2020 - 2030 このレポートでは先進創傷ケア技術の技術市場は2030年には220億ドルに上ると予測されています。糖尿病性足部潰瘍(DFU)、静脈性下腿潰瘍(VLU)、褥瘡(PU)、やけど、その他(外傷性損傷や手術跡)などのセ... もっと見る
出版社
IDTechEx
アイディーテックエックス 出版年月
2019年8月13日
価格
お問い合わせください
ライセンス・価格情報/注文方法はこちら 納期
お問合わせください
ページ数
222
言語
英語
※価格はデータリソースまでお問い合わせください。
サマリー
このレポートでは先進創傷ケア技術の技術市場は2030年には220億ドルに上ると予測されています。糖尿病性足部潰瘍(DFU)、静脈性下腿潰瘍(VLU)、褥瘡(PU)、やけど、その他(外傷性損傷や手術跡)などのセグメントに分けた10年間の市場予測や、この10年間で注目すべき技術開発の動向を掲載しています。
主な掲載内容 ※目次より抜粋
Report Details
Though the human body has a tremendous capability to heal itself, wound healing can stall and create chronic non-healing wounds due to a variety of reasons such as ischemia, bacterial contamination and chronic inflammation.
Common chronic wounds include diabetic foot ulcers (DFU), venous leg ulcers (VLU) and pressure ulcers (PU). Advanced wound care is often required in the treatment of these chronic ulcers - less than 25% of DFUs and less than 30% of VLUs are successfully treated by the current standard of care treatments.
Diabetes, patient age, and obesity are critical risk factors to developing the common non-healing wounds of DFUs, VLUs and PUs. Populations with these risk factors will rise drastically in the next 10 years, especially in comparison to the last 10 years. Thus, the incidence of chronic wounds around the world is expected to rise with the increasing elderly population, as well as the increase in obesity and diabetes. Already there are close to 1 billion persons aged 60 and above, over 2 billion adults who are overweight, and over 500 million persons with diabetes worldwide.
In this report, the market for advanced wound care technologies is forecast to exceed $22 billion by the year 2030. The 10-year forecast is also broken down by the wound types of DFU, VLU, PU, burns and other (for example, traumatic injuries and surgical wounds). Key trends in technological development to watch out for in the next 10 years include:
The report includes a breakdown of the wound care market by wound type.
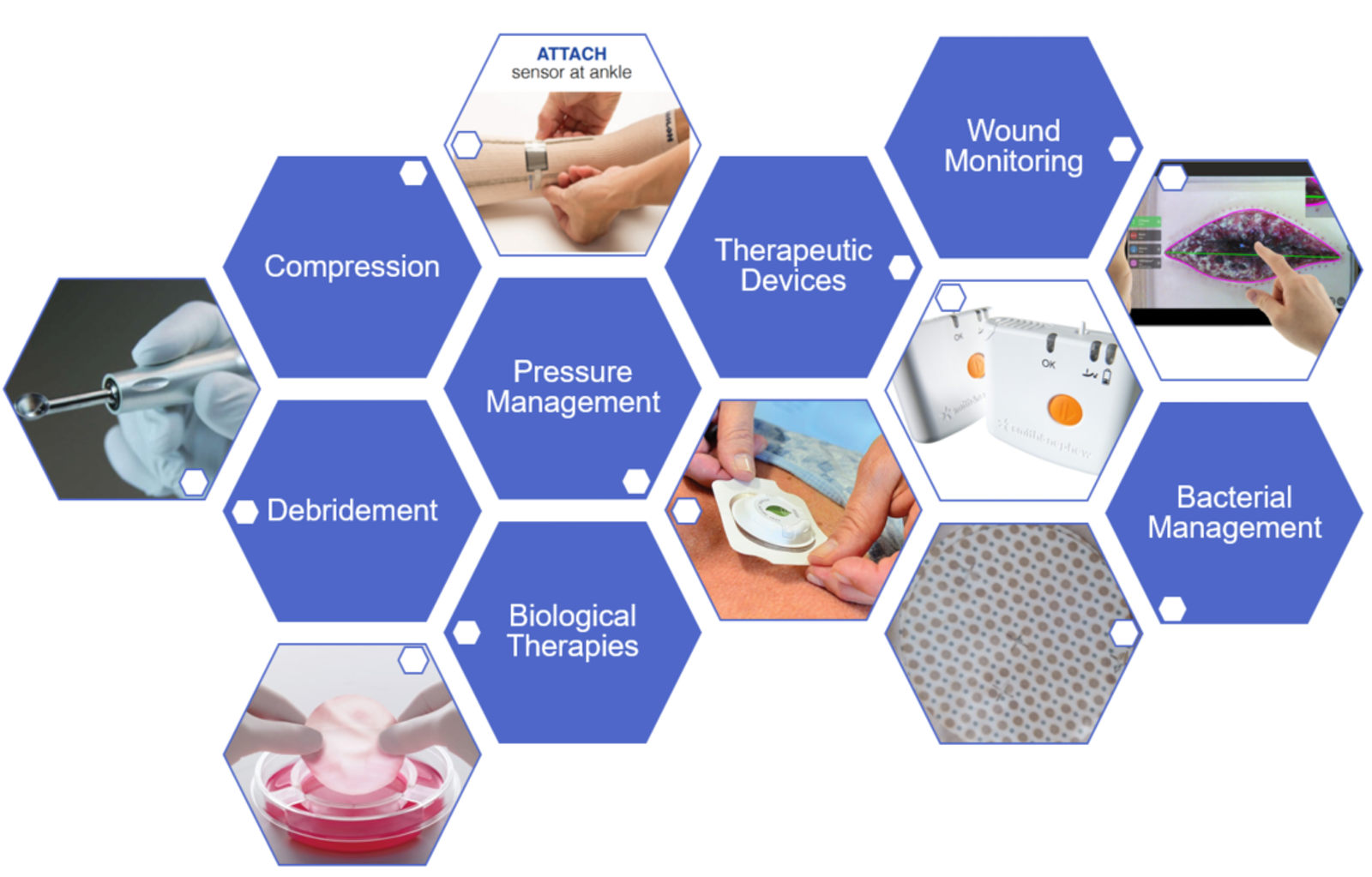
The report covers a broad spectrum of wound care technologies under the following categories:
Examples of technologies covered in this report include ultrasonic probes for debridement, smart beds and shoes for ulcer prevention, sensors for compression pressure monitoring, point-of-care imaging devices for bacterial management, bioelectronic devices for improving healing rates, negative pressure wound therapy with instillation and 3D wound imaging cameras. An entire chapter of the report is dedicated to current state of electronic skin patches for the monitoring of wound status.
Examples of wound care products covered in this report.
目次Table of Contents
Summary
このレポートでは先進創傷ケア技術の技術市場は2030年には220億ドルに上ると予測されています。糖尿病性足部潰瘍(DFU)、静脈性下腿潰瘍(VLU)、褥瘡(PU)、やけど、その他(外傷性損傷や手術跡)などのセグメントに分けた10年間の市場予測や、この10年間で注目すべき技術開発の動向を掲載しています。
主な掲載内容 ※目次より抜粋
Report Details
Though the human body has a tremendous capability to heal itself, wound healing can stall and create chronic non-healing wounds due to a variety of reasons such as ischemia, bacterial contamination and chronic inflammation.
Common chronic wounds include diabetic foot ulcers (DFU), venous leg ulcers (VLU) and pressure ulcers (PU). Advanced wound care is often required in the treatment of these chronic ulcers - less than 25% of DFUs and less than 30% of VLUs are successfully treated by the current standard of care treatments.
Diabetes, patient age, and obesity are critical risk factors to developing the common non-healing wounds of DFUs, VLUs and PUs. Populations with these risk factors will rise drastically in the next 10 years, especially in comparison to the last 10 years. Thus, the incidence of chronic wounds around the world is expected to rise with the increasing elderly population, as well as the increase in obesity and diabetes. Already there are close to 1 billion persons aged 60 and above, over 2 billion adults who are overweight, and over 500 million persons with diabetes worldwide.
In this report, the market for advanced wound care technologies is forecast to exceed $22 billion by the year 2030. The 10-year forecast is also broken down by the wound types of DFU, VLU, PU, burns and other (for example, traumatic injuries and surgical wounds). Key trends in technological development to watch out for in the next 10 years include:
The report includes a breakdown of the wound care market by wound type.
The report covers a broad spectrum of wound care technologies under the following categories:
Examples of technologies covered in this report include ultrasonic probes for debridement, smart beds and shoes for ulcer prevention, sensors for compression pressure monitoring, point-of-care imaging devices for bacterial management, bioelectronic devices for improving healing rates, negative pressure wound therapy with instillation and 3D wound imaging cameras. An entire chapter of the report is dedicated to current state of electronic skin patches for the monitoring of wound status.
Examples of wound care products covered in this report.
Table of ContentsTable of Contents
ご注文は、お電話またはWEBから承ります。お見積もりの作成もお気軽にご相談ください。本レポートと同分野(医療/ヘルスケア)の最新刊レポートIDTechEx社の その他分野 での最新刊レポートよくあるご質問IDTechEx社はどのような調査会社ですか?IDTechExはセンサ技術や3D印刷、電気自動車などの先端技術・材料市場を対象に広範かつ詳細な調査を行っています。データリソースはIDTechExの調査レポートおよび委託調査(個別調査)を取り扱う日... もっと見る 調査レポートの納品までの日数はどの程度ですか?在庫のあるものは速納となりますが、平均的には 3-4日と見て下さい。
注文の手続きはどのようになっていますか?1)お客様からの御問い合わせをいただきます。
お支払方法の方法はどのようになっていますか?納品と同時にデータリソース社よりお客様へ請求書(必要に応じて納品書も)を発送いたします。
データリソース社はどのような会社ですか?当社は、世界各国の主要調査会社・レポート出版社と提携し、世界各国の市場調査レポートや技術動向レポートなどを日本国内の企業・公官庁及び教育研究機関に提供しております。
|
|